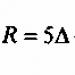Class: 7
Presentation for the lesson






















 Back forward
Back forward
Attention! Slide previews are for informational purposes only and may not represent all the features of the presentation. If you are interested in this work, please download the full version.
Class: 7th grade.
Student age: from 25 years to 30 years
The purpose of the lesson: Introduce into the world of the structure of matter.
Lesson objectives:
Educational:
- Introduce students to the structure of matter.
- Introduce new concepts: “molecule”, “atom”.
- Introduce students to the properties of molecules.
- Show the need to study this topic and use knowledge on the topic in practice.
Developmental:
- Develop students' interest in learning,
- Expand their horizons, memory, imagination.
- Develop the ability to think, draw conclusions, compare, reflect,
- Apply acquired knowledge to a new situation.
- Formation of information competence.
Educational:
- Formation of a scientific picture of the world and worldview among students,
- Continue to develop positive motivation for learning.
- Communication skills, discipline.
- Instill interest in studying physics.
Lesson type: a lesson in learning new material using a presentation.
Equipment: computer, multimedia projector, presentation “Structure of matter. Molecule”, textbook “Physics -7” by A.V.Peryshkin, three beakers, blue gouache, a vessel with water.
During the classes
1. Org. moment.
Repetition of previously studied material:
Test No. 2 on the topic: “Physical phenomena” - self-control.
1. Which of the following is a physical body?
8. hurricane.
2. Which of the following is a substance?
4. pencil
5. rope
8. glass.
3. What words denote physical quantities?
2. speed
4. ruler.
4. What phenomena are considered mechanical?
1. bird flight
2. solar radiation
3. falling raindrops
5. What phenomena are considered physical?
2. yellowed leaves
3. falling raindrops.
| Teacher's activities in the classroom | Student activity |
| 2. Repetition of previously studied material: 1). Working with the dough. 2). Self-control |
1. Students work on the test. 2. Having completed it, check the answers with mine (they are written on the board). 3. Give themselves grades (I will later transfer them to the journal) Right answers - “5” rating – 5 “4” ---------4 “3” ----------3. |
| 3. Presentation of new material: (the teacher’s story is accompanied by a presentation and conversation with students) 1). You and I know that in physics, the bodies surrounding us are stone, the Moon, houses, tables... What is the name of? Slide number 2. 2). What are all physical bodies made of? Slide number 3. 3). What do all substances consist of? (I develop students’ interest, motivate them to a new topic) Slide number 4. |
Answer to the question. Answer to the question. Student answers vary |
| 4). Slide number 5. Write down the topic of the lesson : “Structure of matter. Molecules.” What will we talk about in our lesson today? |
Students write down the topic of the lesson in their notebooks. The students' answers vary, but I bring them to the purpose of the lesson. |
| 5). When asked what all bodies are made of, scientists thought about it in ancient times, because it was necessary to engage in construction: build temples, pyramids, navigation developed, and land was cultivated. And for this it was necessary to know how bodies and substances behave under certain conditions. Information was needed about the properties of various materials, etc. Scientists of that time posed the question: “What are all the bodies around us made of?” What do you think is the importance of this issue? Are they solid or are they built from some very small particles that cannot be seen, but the existence of which, based on observations, they guessed. Slide No. 6, No. 7, No. 8. (about scientists and their statements about the structure of substances). |
Students listen to the teacher. Answers vary from students. Write in a notebook. 2500 years ago Democritus: “All substances are made up of particles.” |
| 6). Slide number 9. Let's consider an experiment with you: A small grain of blue gouache was dissolved in a vessel with water. After some time, the water in it will turn blue. Let's pour some colored water into another vessel and add clean water into it. The solution in the second vessel will be less colored than in the first. Then from the second vessel we again pour the solution into the third vessel and top it up again with clean water. The water in this vessel will be even less colored than in the second vessel. Question: What conclusion can be drawn after this experience? Conclusion: a small grain of gouache was dissolved in water and only a tiny part of it got into the third vessel, then we can assume that the substance consists of very small particles Question: What hypothesis does this experiment confirm? |
Students try to give an answer, i.e. draw a conclusion. Students give an answer. Make a note in a notebook. |
| 7). Slide number 10. We repeat once again: 1. What are physical bodies made of? 2. What does the substance consist of? 3. What do particles of matter consist of? |
|
| 8). And a particle of matter consists of very small particles. This has been proven by modern science. This small particle was called a “molecule”. Question: What is a “molecule”? Slide number 11. |
Entry “Molecule” Notation: a molecule of a substance is the smallest particle of a given substance. |
| 9). Let's imagine the size of the molecule. Slide number 12. Due to their small size, molecules are invisible to the naked eye or a regular microscope. But with the help of a special device - an electron microscope, Slide number 13, You can examine and even photograph larger molecules. F -7, page 19, Fig. 20 photo - arrangement of protein molecules. It is calculated that in a volume of 1 cm 3 of air there are about 27 10 18 molecules. |
Notebook entry: Comparison: molecule – apple – Earth. Recording: electron microscope. Working with the textbook. Write in a notebook. |
| 10). The molecule consists of even smaller particles - atoms. | |
| Slide number 14. The word “atom” means indivisible. You will study the structure of the atom in chemistry lessons in the 8th grade and in physics lessons in the 9th grade. Slide number 15. Atoms are usually designated by symbols: “O” oxygen atom “H” is a hydrogen atom. (See the table of D.I. Mendeleev) The smallest particle of water is a water molecule. It consists of three atoms: two hydrogen atoms and one oxygen atom. |
Atom is “indivisible”. Write in a notebook. |
| Slide number 16. (You will learn about this more fully in chemistry lessons in 8th grade). eleven). In the 18th century, a huge contribution to the development of the doctrine of the structure of matter was made by the Russian scientist Mikhail Vasilyevich Lomonosov - “Physical bodies are divided into the smallest parts.” 12). Question: Why do you think we study the structure of matter? |
They learn that they will study chemistry in 8th grade. Recording: M.V. Lomonosov. They give their answers. |
| Slide number 18. The emergence of ideas about the structure of matter has made it possible not only to explain many phenomena occurring around us, but also to predict how they will occur under certain conditions. It became possible to influence the origin of phenomena, that is, to help control these phenomena. Knowing the structure of bodies, we will answer the questions: why, when heated, do solids turn into liquids, and liquids into gas? Why is rubber elastic and wax soft? Why do two drops of water merge into one, but at the same time two steel balls bounce off each other upon impact? We will answer these and other questions; knowing the structure of matter. |
|
| 4. Consolidation of new material: Slide number 19. No. 1. Look at the slide and answer: Why does a steel ball, which passes freely through the ring, get stuck in the ring after heating? Why can’t the ball pass through the ring, what happened? Yes this is correct. Matter consists of particles with spaces between them. And if the particles move away from each other, then the volume of the body increases. Conversely, when particles come closer together, the volume of the body decreases. |
Students think and give answers to the problem. Draw conclusions about the properties of molecules. Note: there are gaps between the molecules. |
| Slide number 20. No. 2. The hand of the golden statue in the ancient Greek temple, which was kissed by parishioners, has become very noticeably thinner over the decades. The priests are in a panic: Someone stole the gold? Or is this a miracle, a sign? What happened? |
Students respond and express their opinions. |
| Slide number 21. No. 3. Why do my pants wear out? #4: Why do shoes wear out? 5. Lesson reflection: Slide No. 22. 1. What new did you learn in this lesson? 2. Will this be useful to you in life? 3. During the lesson, were you interested in working, listening to the teacher, watching the presentation? 6. Grades for your lesson: 7. Slide number 23. 8. Thanks for the lesson |
Listen to ratings and comments on them. |
Lesson outline: “Structure of matter. Molecules"
(write in student notebooks).

3 See a lot in a little Lesson objectives: 1. know what matter is made of 2. know how the size of molecules was determined 3. know which atoms are most common in the Universe 4. be able to apply knowledge about the structure of matter to solve qualitative problems "One experience I I value above a thousand opinions born of imagination (M.V. Lomonosov)


5 The substance consists of a huge number of tiny particles. A piece of sugar was thrown into the Black Sea, whose area is m 2, depth is 1 km. Now, if we scoop up water anywhere and at any depth, then we will have up to 100 particles of sugar in the bucket

6 Number of particles 1 cm 3 of air 1 cm 3 contains molecules of molecules per second - 9000 years Ball with air 0.007 mm Population of the Earth

7 Particle sizes English physicist John Rayleigh (1842 – 1919) V = 1 mm 3 1 m 2 1 mm mm mm

8 Particle sizes Globe Particle Apple (0, mm) (61 mm) (12742 km)



11 Molecules In 1647, Pierre Gassendi (French physicist) coined the word “molecule”. A molecule is the smallest particle of a substance that retains its chemical properties. Molecules of the same substance are the same, but different substances are different (in size, composition) A molecule consists of atoms The word “molecule” is translated as “small mass”





16 Structure of the atom English scientist Ernest Rutherford () 1. Atomic nucleus (100 thousand times smaller than an atom) 2. Light particles - electrons - move around the nucleus The nucleus consists of particles: protons and neutrons.


18



The following phenomena are known: softening of glass when heated, boiling of water, burning of wood, reaction of marble with hydrochloric acid, glow of an electric light bulb, burning of kerosene, dissolution of sugar in water, freezing of water, change in the shape of a piece of iron during forging, formation of a precipitate when mixing two solutions,
Physical phenomena
Chemical phenomena

From the list below, select those that are chemical and indicate their characteristics:
melting ice, fogging of glass, melting paraffin, burning of forests, freezing of water bodies, rusting of iron, rotting of apples, fermentation of cabbage during pickling
chemical phenomenon
sign




We are surrounded by various items ( body ).
For example:
glass desk ruler
These bodies are made up of substances :
wood
glass
plastic
There are a lot of substances in the world. And they all consist of smallest particles:
ATOMS and MOLECULES

Molecules - these are the smallest particles that make up substances
- Substance molecule - is the smallest particle of a given substance ..
- The smallest particle of water is a water molecule.
- The smallest particle of sugar is a sugar molecule.

ATOM - the smallest chemical
indivisible particle
substances
ATOMS so small
what's on the point of a needle
many of them can fit billions.

Molecular sizes
If molecules became the size of a dot on a sheet of paper. Then all bodies would also increase and the top of the Eiffel Tower would reach the Moon, people would be 1700 km high, mice would be 100 km long, and flies would be 7 km long, each hair would be 100 m thick, the red cells of our blood - red blood cells would have would be 7m in diameter.
H a person is so many times larger than an atom, how many times is it smaller than the star.

- Here's another example: one drop of water contains as many molecules as there are such drops in the Black Sea

- Particles as small as molecules cannot be seen with a simple microscope. However, there are electron microscopes and with their help it became possible to obtain photographs of molecules and even atoms.


Atoms can be seen in the most modern electron microscopes!
carbon
gold
nickel

Composition of a water molecule
Molecules
oxygen molecules
hydrogen
water molecules

Atom is the smallest chemically indivisible particle of a substance.
Atom models:
Molecules are “companies” (groups) of atoms.
Molecule is the smallest particle of a substance that retains its properties.
Molecule models:

Total exists 118 types of atoms (89 in nature)
collection of atoms one type are called
CHEMICAL ELEMENT
Used to represent different atoms
CHEMICAL SIGNS
(letters)

Signs
chemical
elements

Russian name
Pronunciation of the sign
Latin name
aluminum
aluminum
argentum

oxygen

beryllium
beryllium

manganese
manganese

hydrargyrum

silicium


All known elements are placed in a table created by the great Russian scientist
Dmitri Ivanovich Mendeleev :




Myths of the ancient Greeks
Titan – Ti
The metal got its name in honor of the Titans, characters from ancient Greek mythology.

Elements named after celestial bodies or planets
solar system
Selenium (Se) – in honor of the Moon.

Uranus (U) – in honor of Uranus
Neptunium (Np) – in honor of Neptune

Elements named after states
Germanium (Ge) – in honor of Germany
Brandenburg Gate

Gallium (Ga), francium (Fr) – in honor of France
Eiffel Tower

Ruthenium (Ru) No. 44 – in honor of Russia
St Basil's Church

Americium (Am) – in honor of America
Statue of Liberty

Elements named after cities
Hafnium (Hf) – in honor of Copenhagen



Elements named after scientists
Curium (Cm) – in honor of Pierre and Marie Curie



1. The names reflect their most important properties.
Phosphorus Chlorine Bromine
– light-bearing green foul-smelling

2. In honor of celestial bodies.
Selenium Tellurium from Greek. Selena - Moon from Greek. Telluris - Earth

Forms of existence
chemical element:
Simple substances
Complex substances
Free atoms

Forms of existence of the chemical element hydrogen:
Free atoms
Simple substance
Complex substance
water molecules
hydrogen atoms
hydrogen molecules

SUBSTANCES
simple
complex
consist of atoms different chemical elements
consist of atoms
one chemical element

Simple substance
Complex substance
A mixture of two
complex substances
A mixture of simple and complex substances


Substances __________ structures are made up of atoms or ions
Substances of molecular structure consist of _______________

- Substances with a molecular structure are made up of molecules
- Liquids or gases
- Substances of non-molecular structure consist of atoms or ions
- Solids,
low melting point
high melting point

Differences between a simple substance and a chemical element
For example, when they say: “Oxygen is a gas,” it is obvious that they mean a simple substance. Because elements cannot be gases.
"Animals breathe oxygen." There is also substance here. Because it is impossible to breathe the element.
"Oxygen is part of ethyl alcohol." But here oxygen is a chemical element.
"Electrical wires are made of copper." Here copper is a simple substance.
“The human brain requires copper to function.” But here we mean the smallest quantities copper as an element .
"Copper is on the periodic table." Of course, you will not find any pieces of copper substance in the table. This means that a chemical element is clearly meant here. More precisely - chemical element symbol Cu .


- compiled by: physics teacher
- Efimova L. N
- Everything that physical bodies are made of is called substance
- Structure of matter. Molecule.
- Purpose of the lesson: To introduce the world of the structure of matter. Introduce students to the structure of matter. Give an idea of the sizes of molecules.
- Heraclitus said - fire is the primary form of matter.
- Thales argued that the origin of all things on earth is water, from which all things are formed.
- 1. A small grain of blue gouache was dissolved in a vessel with water. The water turned blue. 2. Pour some colored water into the second vessel and add clean water into it. The solution in it will be colored, but weaker.
- 3. From the second vessel, pour the solution into the third vessel and again add clean water. The solution in the third vessel will be colored, but weaker than in the second.
- We dissolved a very small grain of gouache in water and only part of it ended up in the third vessel. This means that the grain itself consisted of a large number of tiny particles This experiment confirms the hypothesis that matter consists of very small particles.
- Physical bodies
- Particles
- Molecules
- Molecule substance is the smallest particle of a given substance.
- Molecule in Latin means "small mass"
- It is calculated that in a volume of 1 cm3 of air there are about 27 1018 molecules.
- Electronic
- microscope
- Molecule
- Atom translated from ancient Greek means “indivisible”.
- "O" is an oxygen atom.
- "H" is a hydrogen atom.
- 2 hydrogen atoms
- 1 oxygen atom
- In the 18th century, the Russian scientist M.V. Lomonosov made a great contribution to the development of the doctrine of the structure of matter.
- “Physical bodies are divided into smallest parts.”
- The emergence of ideas about the structure of matter has made it possible not only to explain many phenomena occurring around us, but also to predict how they will occur under certain conditions.
- It became possible to influence the origin of phenomena, i.e. help manage these phenomena.
- Explain based on Democritus' hypothesis about the existence of tiny particles.
- Why does a steel ball that passes freely through the ring get stuck in the ring after heating?
- The hand of a golden statue in an ancient Greek temple, which was kissed by parishioners, has noticeably lost weight over decades. The priests are in a panic: someone stole the gold? Or is this a miracle, a sign? What happened?
- Why do shoes wear out?
- Why do my pants wear out?
- 1. What new did you learn in class?
- 2. Will this be useful to you in life?
- 3. Was the lesson interesting?